-
Current epidemiologic assessment and Underwriting guidance on COVID-19
The numbers and statements presented in this document are true for 10th March 2020.

-
Updated on 11th March 2020
Dear Client,
At SCOR, we are constantly monitoring the situation relating to the current outbreak of the coronavirus (SARS-CoV-2); the disease of the SARS-CoV-2 infection is now named as coronavirus disease 2019 (COVID-19)” by the World Health Organisation.
This page provides regular updates on the current status of the disease, along with input from our medical doctors, epidemiologist and underwriters providing up to date statistics on incidence and deaths, as well as some overall guidance on the approach to the risk assessment considerations of COVID-19.
Given that data on COVID-19 changes constantly, the numbers and statements presented in this document are true for 5th March 2020. The number of cases in this document are based on official numbers published by the World Health Organization.
Key Highlights
- Since the outbreak of COVID-19 outside of China on January 22, there has been a slow to moderate progression of incidence up to February 21. Since this date there has been a rapid rise in the number of cases notably in Europe, South Korea and Iran with cases rising on a daily basis in most other countries worldwide
- 113,702 confirmed cases were reported globally (80,924 in Greater China) including 4,012 deaths (3,140 in Greater China).
- Italy (9,172 cases) has the second-highest national total behind China and before South Korea (7,513). Cases have rapidly increased in Iran (7,161) as well as France with 1,402 cases in just a few days.
- There is a significant difference in the Case Fatality Ratio (CFR) value across regions. In Hubei, the CFR is as high as 4.46%. In the rest of China it is as low as 0.88% and outside China 2.66%. Italy is currently an outlier with 463 deaths from 9,172 cases (5.05%). This might be due to the specific age structure of the Italian population, with a high number of elderly people, more vulnerable to the disease.
- A study found that the virus has had very few mutations since the beginning of the epidemic, so the probability of the virus becoming more virulent is low.
Background
Since December 2019, a significant number of cases of pneumonia associated with a new coronavirus has been reported in the People's Republic of China. The virus has spread to 109 countries outside of China. On 13th February 2020, the Coronavirus Study Group (CSG) of the International Committee on Taxonomy of Viruses renamed the 2019-nCoV virus as the SARS-CoV-2 virus. The disease due to this virus is now called COVID-19.
Case definition change:
On 13th February, there was a sharp increase in confirmed cases in Hubei province, the center of the epidemic. The definition of confirmed cases has been changed to include suspected cases with positive CT scan results, in addition to patients with positive laboratory test results.
According to the World Health Organization (WHO), a confirmed case should be “a person with laboratory confirmation of 2019-nCoV infection, irrespective of clinical signs and symptoms”. The previous definition in Hubei was in line with the WHO definition. Under the new case definition, patients with all kinds of respiratory infections such as seasonal flu and pneumonia caused by other respiratory viruses, will be classified as confirmed cases of 2019-nCoV infection. This may overestimate the number of cases from Hubei province.
With currently available data, the number of new cases in the rest of China, excluding Hubei, has continued to decrease since 5th February 2020, as shown in the graph below:
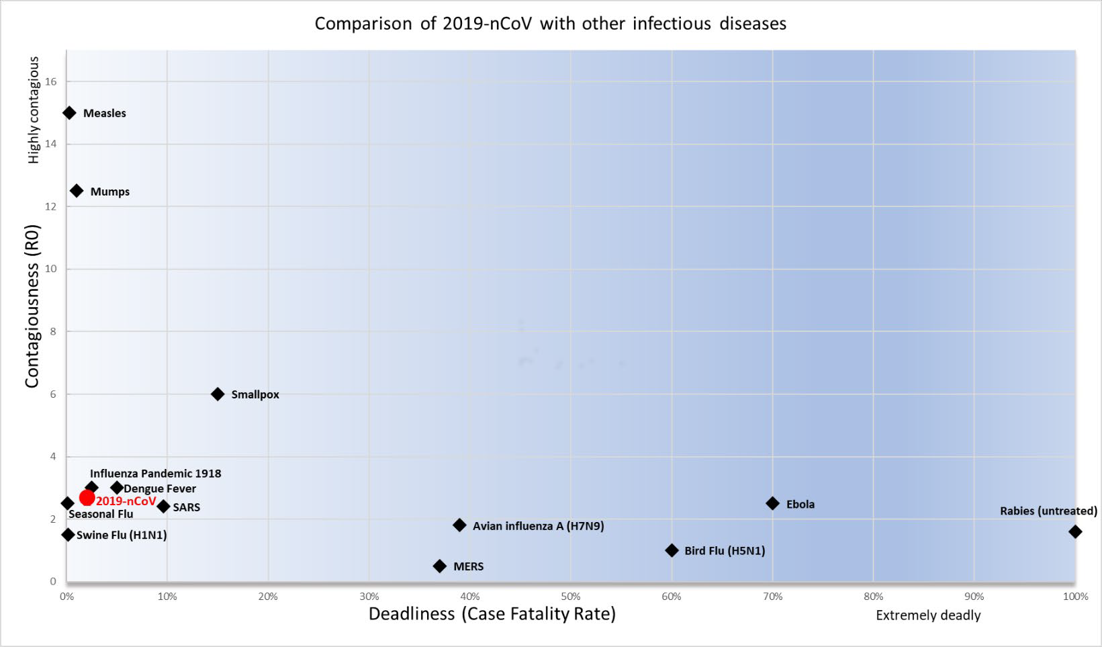
Comparison of 2019-nCOV with other infectious diseasesz Epidemiological characteristics
Based on data reported on 10th March, we observe significant differences in case-fatality rates (CFR) across regions. At the epicentre of the epidemic, Hubei, the CFR is as high as 4.46%, while the rest of China and outside China has a CFR as low as 0.88% and 2.66% respectively. We believe that, due to the limitation of testing capacity, mild cases are less likely to be tested and confirmed in Hubei. At the same time, it is quite likely that severe cases may not be treated promptly because of saturation of health services in Hubei. These two reasons plus the possible high percentage of nosocomial transmission (diseases originating from hospitals), could have led to the higher CFR in Hubei.
Reported laboratory-confirmed COVID 19 cases and deaths- Date as 10th March
Reported laboratory-confirmed COVID 19 cases and deaths- Date as 10th March Region Total number
of casesNumber of new cases in the last 24 hours Total number
of deathCFR China 80,924 20 3,140 3.88% Hubei, China* 67,760 17 3,024 4.46% China, excluding Hubei 13,164 3 116 0.88% Outside China 32,778 4,105 872 2.66% Italy 9,172 1,797 463 5.05% Republic of Korea 7,513 131 54 0.72% Iran 7,161 595 237 3.31% France 1,402 286 30 2.14% Germany 1,139 27 2 0.18% Spain 1,024 435 28 2.73% Japan 514 26 9 1.75% USA 472 259 19 4.03% UK 323 46 3 0.93% A recent study18 estimated the CRF stratified by age, sex and disease severity, based on patient data reported to the Chinese Center For Disease Control (CDC) up to 26th January 2020. The result shows that male patients over 60 years old have substantially elevated CFR. Patients who developed severe pneumonia also have a higher CFR, and the risk is further increased in older males. The authors suggested that the higher prevalence of virus receptors in the lungs of Asian males could be the reason for gender differences observed in the CFR.
Genetic variation
In another recent study19, scientists found that SARS-CoV-2 evolves at a rate compatible with related coronaviruses (approximately 0.0007- 0.002 genetic substitutions per site per year), and they concluded that the very short period of observation has allowed very few mutations to occur.
Mitigation measures
The Chinese government has taken significant measures including the shutdown of big cities, public transport and limiting public gatherings. The efficacity of these measures has started to be seen since 6th Feb when new case numbers decreased.
Countries across the European region continue to prepare for and respond to cases of COVID-19. This includes establishing how to promptly detect sick people, testing samples from suspect cases, ensuring appropriate infection control and case management to minimize the risk of the virus spreading, and maintaining communication with the public. On 10th March Italy has even extended strict quarantine measures across the whole country.
Mitigation measures have for goal to slow acceleration of number of cases, to reduce peak number of cases and related demands on hospitals and infrastructure, reduce the number of overall cases and health consequences.
Treatment and vaccines
Currently there is no specific antiviral treatment available for SARS-CoV-2, hence patients are only receiving supportive care. However, some previously developed antiviral drugs that have shown activity against coronavirus in vitro are beginning to be tested in clinical settings:
- A clinical trial with a new antiviral drug remdesivir (Gilead) has started in China which has shown "in vitro and in vivo activity in animal models against viral pathogens", including MERS SARS and 2019-nCoV.
- Thai Physicians claimed that they have had good clinical results with a combination of antivirals treatment (anti-flu and anti-HIV).
- A clinical trial with Lopinavir/ritonavir (Kaletra ™) has started in China. Kaletra ™ is a protease inhibitor used in highly active antiretroviral therapy (HAART) for the management of HIV/AIDS.
- Treatment of 5 patients in Singapore with lopinavir-ritonavir resulted in 3 showing improvement and 2 deteriorating. There were no deaths.
There are currently 329 clinical trials on treatment for SARS-CoV-2 registered on the WHO clinical trial platform. Four vaccine candidates are in the pre-clinical phase of development
Risk assessment for underwriters
In terms of SCOR’s guidance for underwriting applications including mortality, critical Illness, disability benefits and medical expenses, we would encourage a cautious approach and adhere to robust risk management principles when assessing risks linked with possible exposure to COVID-19.
- Life Cover: High impact mainly for people aged above 60 years and people with co-morbidities
- Critical Illness: Not all products will be impacted. High incidence of respiratory failure, kidney failure and multiple organ failure, critical or intensive care benefits and heart attack after the COVID-19 disease.
- Disability benefits: May be impacted by a high incidence of kidney failure and after-effects following critical care treatment.
- Medical expenses: Impact depends on public health services in each country. If the cost of the treatment is covered by the government, minimal to no impact is expected.
Any applicant with flu-like symptoms or recent referral to a hospital or clinic for similar symptoms should be cautiously underwritten, especially in countries where COVID-19 has been reported.
For those applicants with recent travel history to affected regions, it is best to follow local governmental and/or WHO guidelines on travel advice to det appropriate underwriting approach.
It has been two months since the outbreak started and a reasonable proportion of the infected population has recovered. For those who have recovered from COVID-19, we recommend waiting at least three months before such applicants are considered for insurance. This is mainly due to unknown short-term outcomes for the COVID-19.
Finally, we would recommend reviewing the medical questions as part of the application process to include the need to identify any risks linked to COVID-19.
As situation is continuously evolving each day with countries now reporting community spread, we believe companies should remain alert on claims related practices and processes to ensure smooth and timely adjudication of claims related to COVID-19.
Your SCOR Underwriting and Knowledge teams remain available to assist you further by providing advice on best practice and sound risk management Underwriting principles. The teams will also ensure that we find the right balance between sound risk management and the need to provide the necessary cover to all potential and existing policyholders.
A perspective on COVID-19 from the United-States
By Doctor Braun, VP Chief medical officer at SCOR.
As of March 5, 2020 the US appears on the cusp of community spread (cases appearing without known source contacts). Confirmed or pending cases have arisen in California, Oregon, Washington, and 11 other states. There are reports of an outbreak in a long-term care facility in Washington state and multiple deaths have occurred. Eleven deaths in total have occurred in the US. New cases were reported in New Hampshire, Illinois, and Oregon on Monday. The situation is fluid and changing rapidly.
The Centers for Disease Control and Prevention (CDC) is preparing communities for possible closure of schools and cancellation of public gatherings. They are also advising on ways to minimize the risk of contacting the virus. Companies are preparing employees for working from home and debating restricting travel plans. While the CDC says that the risk to the average American remains low, they also say that more community spread and more deaths will be occurring.
Quarantines, travel restrictions, and prevention of public gatherings appears to be working in China as they report fewer and fewer new cases daily. As a bit of perspective, according to the Worldbank, the population of China is approximately 1.4 billion people. As of today, 3,140 deaths due to COVID-19 have been reported in China, and as stated earlier, new cases appear to be on the wane.
In the US the outcome will largely depend on the public’s willingness to follow direction from federal, state and local governments or from personal efforts to prevent the spread of infection. Medications are under study, and vaccines under development, but deaths are still occurring, mainly in the elderly and those with co-morbid conditions.
If extra caution is desired during underwriting, consider a statement of good health on delivery. As far as increased buying activity in a region of community spread, this may turn out to be a net positive. Anyone knowledgeable enough to realize the risk will no doubt be taking measures to mitigate it. And it may have the overall effect of making people consider their own mortality, resulting in increased sales in the long run.
It is early in the course in the US, but the life industry will no doubt continue to provide protection to their clients through and beyond this outbreak.
Bibliography:
1) WHO. Preliminary assessment of the International Spreading Risk Associated with the 2019 novel Coronavirus (2019-nCoV) outbreak in Wuhan City
2) WHO. Novel Coronavirus (2019-nCoV) SITUATION REPORT 1 – 8, 29 JANUARY 2020
3) Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. bioRxiv. http://dx.doi.org/10.1101/2020.01.22.914952
4) ECDC. Outbreak of acute respiratory syndrome associated with a novel coronavirus, China; First cases imported in the EU/EEA; second update. 26 January 2020
5) Wu Peng, Hao Xinxin, Lau Eric H Y, Wong Jessica Y, Leung Kathy S M, Wu Joseph T, Cowling Benjamin J, Leung Gabriel M. Real-time tentative assessment of the epidemiological characteristics of novel coronavirus infections in Wuhan, China, as at 22 January 2020. Euro Surveill. 2020;25(3):pii=2000044. https://doi.org/10.2807/1560-7917.ES.2020.25.3.2000044
6) Li Q et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020 Jan 29. doi: 10.1056/NEJMoa2001316. [Epub ahead of print] https://doi.org/10.1016/S0140-6736(20)30251-8
7) Guan W. et al. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv 2020.02.06.20020974; doi: https://doi.org/10.1101/2020.02.06.20020974
8) ECDC. Current risk assessment on the novel coronavirus situation, 10 February 2020
9) ECDC. https://www.ecdc.europa.eu/sites/default/files/documents/novel-coronavirus-risk-assessment- china-31-january-2020_0.pdf
10) World Health Organization (WHO). Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV) 2020 [updated 23 January 2020]. https://www.who.int/news-room/detail/23-01-2020- statement-on-the-meeting-of-the-international-health-regulations-(2005)-emergency-committee- regarding-the-outbreak-of-novel-coronavirus-(2019-ncov)
11) Wu JT et al. Nowcasting and forecasting the potential domestic and international spread of the 2019- nCoV outbreak originating in Wuhan, China: a modelling study. Published Online January 31, 2020 https://doi.org/10.1016/S0140-6736(20)30260-9.
12) Tuite AR, Fisman DN . Reporting, Epidemic Growth, and Reproduction Numbers for the 2019 Novel Coronavirus (2019-nCoV) Epidemic Free. Ann Intern Med. 2020. DOI: 10.7326/M20-0358
13) Report 4: Severity of 2019-novel coronavirus (nCoV). Dorigatti I et al. WHO Collaborating Centre for Infectious Disease Modelling. MRC Centre for Global Infectious Disease Analysis. J-IDEA. Imperial College London
14) ECDC. Current risk assessment on the novel coronavirus situation, 11 February 2020. https://www.ecdc.europa.eu/en/current-risk-assessment-novel-coronavirus-situation
15) Backer Jantien A , Klinkenberg Don , Wallinga Jacco . Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20–28 January 2020. Euro Surveill. 2020;25(5):pii=2000062. https://doi.org/10.2807/1560-7917.ES.2020.25.5.2000062
16) WHO. List of candidate vaccines under development against nCoV. https://www.who.int/blueprint/priority-diseases/key-action/list-of-candidate-vaccines-developed- against-ncov.pdf?ua=1
17) WHO. International Clinical trials registered platform. http://apps.who.int/trialsearch/AdvSearch.aspx?SearchTermStat=117&ReturnUrl=~/ListBy.aspx?TypeLi sting=0
18) Yang et al., Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China. medRxiv preprint doi: https://doi.org/10.1101/2020.02.10.20021675
19) Report 5: Phylogenetic analysis of SARS-CoV-2. Volz et al. WHO Collaborating Centre for Infectious Disease Modelling. MRC Centre for Global Infectious Disease Analysis. J-IDEA. Imperial College London
20) Young BE, Ong SW, Kalimuddin S. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA. Published online March 3, 2020. Doi:10.1001/jama.2020.3204.
APPENDIX 1. Comparison of COVID-19 with other infectious diseases
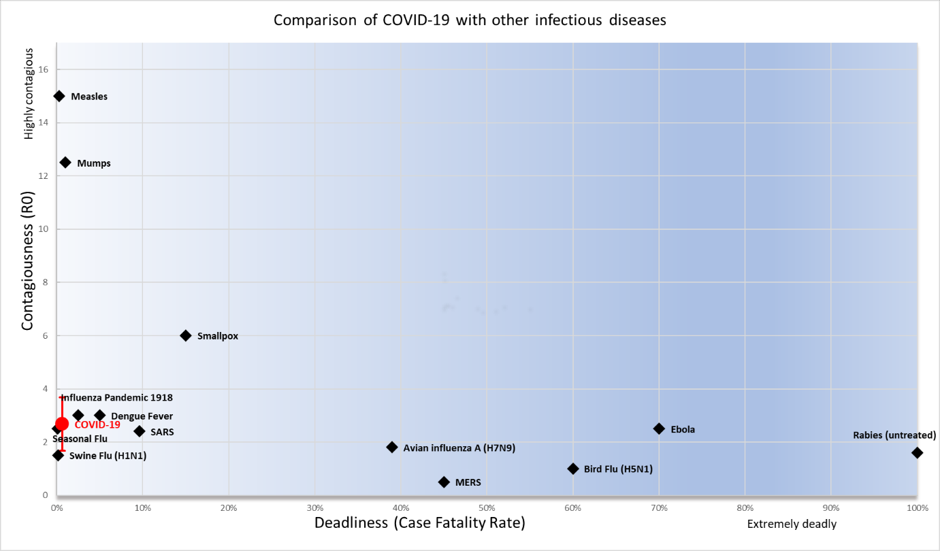
Comparison of COVID-19 with other infectious diseases The Case Fatality Rate (CFR) is defined as the proportion of cases of a disease who will ultimately die from the disease. For a given case definition, once all deaths and cases have been ascertained (for example at the end of an epidemic), this is simply calculated as deaths/cases. However, at the start of the epidemic this ratio is unstable due to (a) underestimations of the true CFR due to the time-lag between onset of symptoms and death and (b)overestimations due to the large number of undetected/untested cases.
